Abstract
Background: BCR-ABL tyrosine kinase inhibitors (TKI) can provide symptom control and improve survival in patients with Ph+ chronic myeloid leukemia (CML), however, elimination of leukemic stem cells (LSC) remains a significant unmet medical need. Persisting LSCs contribute to resistance and disease relapse, which can occur in approximately 50% of patients who elect to discontinue TKIs after sustained deep molecular responses (Cortes et al. Am J Hematol. 2018). Furthermore, patients in accelerated or blast phase have lower response rates, shorter response duration, and inferior survival outcomes. Patients who are not eligible for stem cell transplant or other TKIs after failure of at least 2 prior TKIs are especially in need of effective treatment options.
In CML cell lines, activation of the BCR-ABL pathway enhances malignant cell survival by upregulating the anti-apoptotic BCL-2 proteins, Mcl-1 and Bcl-X L, and MDM2, a key negative regulator of the tumor suppressor protein, p53. Mechanistically, TKIs induce apoptosis of CML cells by suppressing these prosurvival pathways (Wendel et al. PNAS. 2006). In ex vivo studies, CD34+ cells from CML patients who developed TKI resistance maintained limited sub-therapeutic suppression of the BCL-2 family proteins (Carter et al. Oncotarget. 2015) and the MDM2 protein.
MDM2 is overexpressed in CD34+ CML cells (Trotta et al. Cancer Cell. 2003) and attenuates p53 activity resulting in the proliferation of malignant CD34+ cells. In cell culture models, the combination of MDM2 inhibitors with TKIs synergistically induced cell death of proliferating and quiescent TKI-insensitive CD34+ CML cells (Carter et al. Oncotarget. 2015). In patient-derived xenograft models, this combination effectively eradicated CML LSCs in the peripheral blood, spleen, and bone marrow (Scott et al. HemaSphere. 2018).
Navtemadlin (KRT-232) is a potent, selective, orally available MDM2 inhibitor that restores p53 function and activates the proapoptotic proteins Bax, Bak, PUMA, and Noxa. Combining navtemadlin with BCR-ABL TKIs has the potential to deliver disease-modifying effects in patients with relapsed/refractory (R/R) CML by inhibiting prosurvival signals, reducing leukemic burden, and targeting LSCs. The low rates of TP53 mutations or loss in patients with CML further supports the rationale to test this combination in R/R CML.
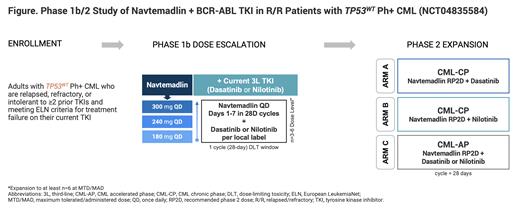
Methods: Navtemadlin in combination with dasatinib or nilotinib is being evaluated in a multicenter, open-label, phase 1b/2 study in patients with R/R CML (NCT04835584). Enrolled patients aged ≥18 y will have documented TP53WT, Ph+, chronic (CP) or accelerated phase (AP) CML and ECOG performance status ≤2. Patients are relapsed/refractory/intolerant to ≥2 prior TKIs and have met the criteria for treatment failure on their current TKI; they should be on stable doses of a BCR-ABL TKI for ≥4 weeks prior to enrollment. Patients with CML in blast phase or with TP53 or BCR-ABL/T315I mutations, or those with prior MDM2 inhibitor treatment will be excluded.
The phase 1b portion of the study will follow a 3+3 dose-escalation design to identify the maximum tolerated dose (MTD) of navtemadlin in combination with the dasatinib or nilotinib (primary endpoint). Up to 18 patients with CML-CP will receive oral navtemadlin once daily (QD) on days (d) 1-7 of 28-d cycles at 180, 240, and 300 mg doses with the pre-study stable dose of dasatinib or nilotinib (Figure). The MTD will be defined as the highest dose level at which <33% of patients experience a treatment-related dose-limiting toxicity. If the MTD is not reached, the maximum administered dose (MAD) will be the highest evaluated dose. The secondary endpoint is the pharmacokinetics of navtemadlin in combination with TKIs.
The phase 2 dose expansion will evaluate the recommended phase 2 dose (RP2D) of navtemadlin from phase 1b in combination with dasatinib (Arm A) or nilotinib (Arm B) in patients with CML-CP. Arm C will evaluate navtemadlin at the RP2D with dasatinib or nilotinib in patients with CML-AP. The primary endpoints of phase 2 are the rate of major cytogenetic response at 6 months in Arms A and B and major hematologic response in Arm C. Secondary endpoints include duration of response, rate of complete hematologic response, progression-free and overall survival, and safety. This trial is ongoing and will enroll patients at approximately 45 global sites in North America, Europe, and Asia.
Cortes: Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sun Pharma: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Ortí: Incyte: Consultancy, Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Pfizer: Consultancy, Honoraria, Other: travel, accommodations, expenses, Speakers Bureau. Prosper: Oryzon: Honoraria; Janssen: Honoraria; BMS-Celgene: Honoraria, Research Funding. Uyei: Kartos Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company; Genentech/Roche: Ended employment in the past 24 months; Gilead Sciences: Current equity holder in publicly-traded company; Telios Pharma: Current holder of individual stocks in a privately-held company. Rothbaum: Quogue Capital: Current Employment; Iovance Biotherapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses; Acerta Pharma/Astra Zeneca: Current equity holder in publicly-traded company; Kartos Therapeutics: Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Patents & Royalties; Telios Pharma: Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Patents & Royalties; Quogue IP Holdings: Patents & Royalties. Nicolini: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Research Funding; Incyte Biosciences: Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; BMS: Honoraria; Kartos Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sun Pharma Ltd.: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Yes, these investigational agents are being evaluated in a clinical trial setting.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal